Atom Model Bohr Čerstvé
Atom Model Bohr Čerstvé. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.
Tady Niels Bohr Atom Model Mobile Von Flensted Mobiles Online Kaufen Bei Nordicnest De
Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.
It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.

It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei... It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.

It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.

It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …

It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a ….. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …

Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …

Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.

It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.

It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron... It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …

It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a ….. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.

It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron... Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.

It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.
It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron... It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.

It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.

It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.

Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …

It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a ….. .. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.

Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …

Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.

Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …

It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.
.jpg)
Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. . It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.

Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …

It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.
It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …

It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …

Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron... It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.

It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …

It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron... It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.

It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.

It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.
Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …

Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …
Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …
It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.

Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei... Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.

It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron... Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.

It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …

Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …

It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.

It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …

It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a ….. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.

Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.

Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.
It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.
It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …

It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …

Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.

It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …

It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.

It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …

Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …

It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.

Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.

It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …

It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …

It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.
.jpg)
It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.

It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …
Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …

Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …

Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.

It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.

It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …

It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.

Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.

It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron... It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.
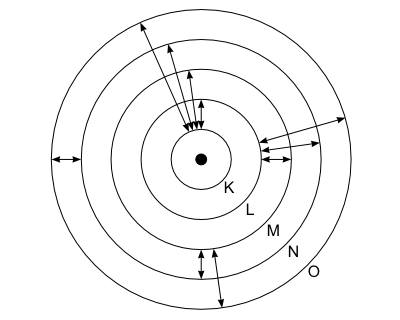
Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.

It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.

It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei... It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …

It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.

It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron... It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …

It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.

Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a ….. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.
Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.

Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …
It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron... Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.

It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.

It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …

Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei... It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.

Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron... Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.
It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. . It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.

Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron... It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …

Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. .. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.

It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.

Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …

Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.

It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.

Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a …
It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron... It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a … Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.