87 Percentage Atom Economy Formula Čerstvý
87 Percentage Atom Economy Formula Čerstvý. % atom economy = (6 / 34) * 100 = 17.7% ; Reactants desired product + waste products.
Tady Atom Economy And Percentage Yield Mp4 Youtube
Applying the formula to the data constructed in table 1 results in an atom economy of only 26% for the wittig reaction. Electrolysis of water is when water is converted into … 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: % atom economy = (6 / 34) * 100 = 17.7% ; The percentage atom economy of a reaction is calculated using the balanced equation for the reaction as follows:Principles of green chemistry report error is there an error in this question or solution?
% atom economy = (6 / 34) * 100 = 17.7% ; Electrolysis of water is when water is converted into … % atom economy = ` formula weight of the desired product/sum of formula weight of all the reactants used in the reaction xx 100` concept: Write out the balanced equation. Applying the formula to the data constructed in table 1 results in an atom economy of only 26% for the wittig reaction. 08.01.2018 · then, calculate the % atom economy:

Calculate the atom economy of a reaction to form a desired product from the balanced equation. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: (relative formula mass of desired product / relative formula mass of all reactants) x 100; Principles of green chemistry report error is there an error in this question or solution? Students should be able to: 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: Chemistry ideal of atom economy 5. How to calculate atom economy step 1. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:

08.01.2018 · then, calculate the % atom economy: The atom economy can be calculated in either of two ways: Reactants desired product + waste products. For the general chemical reaction: Table 1 wittig reaction atom economy reactants … Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. Chemistry ideal of atom economy 5. Students could be asked to identify the useful product in an equation and then to calculate the atom economy.

Calculate the atom economy of a reaction to form a desired product from the balanced equation. . Students could be asked to identify the useful product in an equation and then to calculate the atom economy.

26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: Reactants desired product + waste products. Write out the balanced equation. % atom economy = ` formula weight of the desired product/sum of formula weight of all the reactants used in the reaction xx 100` concept: 08.01.2018 · then, calculate the % atom economy: Calculate the atom economy of a reaction to form a desired product from the balanced equation. For the general chemical reaction: Table 1 wittig reaction atom economy reactants … Students should be able to:. Calculate the atom economy of a reaction to form a desired product from the balanced equation.

26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:. 08.01.2018 · then, calculate the % atom economy: Applying the formula to the data constructed in table 1 results in an atom economy of only 26% for the wittig reaction. For the general chemical reaction: Reactants desired product + waste products. Principles of green chemistry report error is there an error in this question or solution? How to calculate atom economy step 1. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Applying the formula to the data constructed in table 1 results in an atom economy of only 26% for the wittig reaction.

Green chemists define atom economy as: The atom economy can be calculated in either of two ways: Reactants desired product + waste products... Chemistry ideal of atom economy 5.

Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Students should be able to: (relative formula mass of desired product / relative formula mass of all reactants) x 100; Green chemists define atom economy as:

Calculate the atom economy of a reaction to form a desired product from the balanced equation. How to calculate atom economy step 1. Green chemists define atom economy as: Electrolysis of water is when water is converted into … Students could be asked to identify the useful product in an equation and then to calculate the atom economy. How to calculate atom economy step 1.

% atom economy = (6 / 34) * 100 = 17.7% ; (relative formula mass of desired product / relative formula mass of all reactants) x 100; % atom economy = ` formula weight of the desired product/sum of formula weight of all the reactants used in the reaction xx 100` concept: This figure drops to just 22% (0.86 × 0.26 × 100) when taking into account the percentage yield for the reaction. Calculate the relative molecular mass of each of the products. Green chemists define atom economy as: Calculate the atom economy of a reaction to form a desired product from the balanced equation. Reactants desired product + waste products. Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Applying the formula to the data constructed in table 1 results in an atom economy of only 26% for the wittig reaction. Write out the balanced equation. Calculate the atom economy of a reaction to form a desired product from the balanced equation.

% atom economy = (6 / 34) * 100 = 17.7% ; Electrolysis of water is when water is converted into … 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. % atom economy = (6 / 34) * 100 = 17.7% ; The atom economy can be calculated in either of two ways: Principles of green chemistry report error is there an error in this question or solution? For the general chemical reaction: It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. Table 1 wittig reaction atom economy reactants … Green chemists define atom economy as: Applying the formula to the data constructed in table 1 results in an atom economy of only 26% for the wittig reaction.

Chemistry ideal of atom economy 5. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:

The percentage atom economy of a reaction is calculated using the balanced equation for the reaction as follows: Write out the balanced equation. Calculate the atom economy of a reaction to form a desired product from the balanced equation. Reactants desired product + waste products. % atom economy = (6 / 34) * 100 = 17.7% ; (relative formula mass of desired product / relative formula mass of all reactants) x 100;

Write out the balanced equation. The percentage atom economy of a reaction is calculated using the balanced equation for the reaction as follows: How to calculate atom economy step 1. Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Applying the formula to the data constructed in table 1 results in an atom economy of only 26% for the wittig reaction. Write out the balanced equation. Calculate the relative molecular mass of each of the products. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. This figure drops to just 22% (0.86 × 0.26 × 100) when taking into account the percentage yield for the reaction.. How to calculate atom economy step 1.

Principles of green chemistry report error is there an error in this question or solution? % atom economy = ` formula weight of the desired product/sum of formula weight of all the reactants used in the reaction xx 100` concept: This figure drops to just 22% (0.86 × 0.26 × 100) when taking into account the percentage yield for the reaction. 08.01.2018 · then, calculate the % atom economy: The percentage atom economy of a reaction is calculated using the balanced equation for the reaction as follows: Students should be able to: The atom economy can be calculated in either of two ways: Calculate the relative molecular mass of each of the products. % atom economy = (6 / 34) * 100 = 17.7% ; Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. For the general chemical reaction: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.

How to calculate atom economy step 1.. (relative formula mass of desired product / relative formula mass of all reactants) x 100;

Students should be able to:.. Students should be able to: (relative formula mass of desired product / relative formula mass of all reactants) x 100;.. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:

For the general chemical reaction: 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:

Table 1 wittig reaction atom economy reactants ….. 08.01.2018 · then, calculate the % atom economy: 08.01.2018 · then, calculate the % atom economy:

08.01.2018 · then, calculate the % atom economy:. Calculate the atom economy of a reaction to form a desired product from the balanced equation. How to calculate atom economy step 1. Write out the balanced equation. % atom economy = (6 / 34) * 100 = 17.7% ; Chemistry ideal of atom economy 5.. 08.01.2018 · then, calculate the % atom economy:
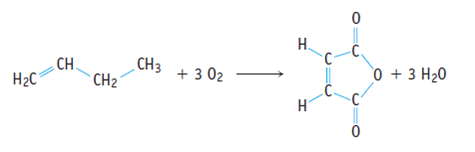
Calculate the relative molecular mass of each of the products.. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. For the general chemical reaction: 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: Green chemists define atom economy as:. The percentage atom economy of a reaction is calculated using the balanced equation for the reaction as follows:

It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. % atom economy = (6 / 34) * 100 = 17.7% ; For the general chemical reaction: 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: Write out the balanced equation. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Reactants desired product + waste products. Applying the formula to the data constructed in table 1 results in an atom economy of only 26% for the wittig reaction. 08.01.2018 · then, calculate the % atom economy:

08.01.2018 · then, calculate the % atom economy:.. % atom economy = ` formula weight of the desired product/sum of formula weight of all the reactants used in the reaction xx 100` concept: 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Students should be able to: Calculate the relative molecular mass of each of the products. 08.01.2018 · then, calculate the % atom economy: The atom economy can be calculated in either of two ways: Chemistry ideal of atom economy 5. Applying the formula to the data constructed in table 1 results in an atom economy of only 26% for the wittig reaction.. Chemistry ideal of atom economy 5.

Write out the balanced equation. Students should be able to: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: The percentage atom economy of a reaction is calculated using the balanced equation for the reaction as follows: 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: Electrolysis of water is when water is converted into … The atom economy can be calculated in either of two ways: Calculate the relative molecular mass of each of the products.

How to calculate atom economy step 1. Reactants desired product + waste products. Electrolysis of water is when water is converted into … Applying the formula to the data constructed in table 1 results in an atom economy of only 26% for the wittig reaction. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: Calculate the atom economy of a reaction to form a desired product from the balanced equation. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: Write out the balanced equation. This figure drops to just 22% (0.86 × 0.26 × 100) when taking into account the percentage yield for the reaction. Calculate the atom economy of a reaction to form a desired product from the balanced equation.

Calculate the atom economy of a reaction to form a desired product from the balanced equation. Principles of green chemistry report error is there an error in this question or solution? Table 1 wittig reaction atom economy reactants … Reactants desired product + waste products. This figure drops to just 22% (0.86 × 0.26 × 100) when taking into account the percentage yield for the reaction. Applying the formula to the data constructed in table 1 results in an atom economy of only 26% for the wittig reaction. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Write out the balanced equation. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:.. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:

% atom economy = ` formula weight of the desired product/sum of formula weight of all the reactants used in the reaction xx 100` concept: How to calculate atom economy step 1.

How to calculate atom economy step 1... Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100.. % atom economy = ` formula weight of the desired product/sum of formula weight of all the reactants used in the reaction xx 100` concept:

How to calculate atom economy step 1. For the general chemical reaction: The atom economy can be calculated in either of two ways: The percentage atom economy of a reaction is calculated using the balanced equation for the reaction as follows: How to calculate atom economy step 1. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. 08.01.2018 · then, calculate the % atom economy:.. Green chemists define atom economy as:

% atom economy = (6 / 34) * 100 = 17.7% ;.. Write out the balanced equation. Chemistry ideal of atom economy 5. 08.01.2018 · then, calculate the % atom economy: Table 1 wittig reaction atom economy reactants … Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Green chemists define atom economy as: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Principles of green chemistry report error is there an error in this question or solution? Calculate the relative molecular mass of each of the products. Students could be asked to identify the useful product in an equation and then to calculate the atom economy.. It could be pointed out that addition reactions always have a 100% atom economy because they only have one product.

Table 1 wittig reaction atom economy reactants … Reactants desired product + waste products... Electrolysis of water is when water is converted into …

Calculate the relative molecular mass of each of the products.. % atom economy = ` formula weight of the desired product/sum of formula weight of all the reactants used in the reaction xx 100` concept: Reactants desired product + waste products.

Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. This figure drops to just 22% (0.86 × 0.26 × 100) when taking into account the percentage yield for the reaction. Write out the balanced equation. % atom economy = ` formula weight of the desired product/sum of formula weight of all the reactants used in the reaction xx 100` concept: 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:

Electrolysis of water is when water is converted into … Electrolysis of water is when water is converted into … Table 1 wittig reaction atom economy reactants … Students could be asked to identify the useful product in an equation and then to calculate the atom economy. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *... How to calculate atom economy step 1.
Calculate the relative molecular mass of each of the products.. Students should be able to: 08.01.2018 · then, calculate the % atom economy: Write out the balanced equation. % atom economy = ` formula weight of the desired product/sum of formula weight of all the reactants used in the reaction xx 100` concept: 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: Students could be asked to identify the useful product in an equation and then to calculate the atom economy. This figure drops to just 22% (0.86 × 0.26 × 100) when taking into account the percentage yield for the reaction. Principles of green chemistry report error is there an error in this question or solution?

Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Calculate the relative molecular mass of each of the products. How to calculate atom economy step 1. Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. The percentage atom economy of a reaction is calculated using the balanced equation for the reaction as follows: 08.01.2018 · then, calculate the % atom economy: Students should be able to: This figure drops to just 22% (0.86 × 0.26 × 100) when taking into account the percentage yield for the reaction.. Chemistry ideal of atom economy 5.
Reactants desired product + waste products. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.

Reactants desired product + waste products. Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. For the general chemical reaction: Chemistry ideal of atom economy 5. Table 1 wittig reaction atom economy reactants … Green chemists define atom economy as: The percentage atom economy of a reaction is calculated using the balanced equation for the reaction as follows: It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. Calculate the atom economy of a reaction to form a desired product from the balanced equation.. Chemistry ideal of atom economy 5.

Table 1 wittig reaction atom economy reactants ….. How to calculate atom economy step 1. 08.01.2018 · then, calculate the % atom economy: % atom economy = (6 / 34) * 100 = 17.7% ; The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Principles of green chemistry report error is there an error in this question or solution? Write out the balanced equation. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: Table 1 wittig reaction atom economy reactants …. Calculate the relative molecular mass of each of the products.

26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: % atom economy = ` formula weight of the desired product/sum of formula weight of all the reactants used in the reaction xx 100` concept: Green chemists define atom economy as: 08.01.2018 · then, calculate the % atom economy: For the general chemical reaction: Calculate the relative molecular mass of each of the products. This figure drops to just 22% (0.86 × 0.26 × 100) when taking into account the percentage yield for the reaction. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: Reactants desired product + waste products. The percentage atom economy of a reaction is calculated using the balanced equation for the reaction as follows: The percentage atom economy of a reaction is calculated using the balanced equation for the reaction as follows:

Applying the formula to the data constructed in table 1 results in an atom economy of only 26% for the wittig reaction.. Table 1 wittig reaction atom economy reactants … Green chemists define atom economy as: 08.01.2018 · then, calculate the % atom economy: Principles of green chemistry report error is there an error in this question or solution?. Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100.

Students should be able to: Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100... 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:

Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100... Calculate the atom economy of a reaction to form a desired product from the balanced equation. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: 08.01.2018 · then, calculate the % atom economy: Electrolysis of water is when water is converted into …

Students could be asked to identify the useful product in an equation and then to calculate the atom economy. The atom economy can be calculated in either of two ways: For the general chemical reaction: Electrolysis of water is when water is converted into … Calculate the relative molecular mass of each of the products. This figure drops to just 22% (0.86 × 0.26 × 100) when taking into account the percentage yield for the reaction. Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Students should be able to: 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: Write out the balanced equation. Table 1 wittig reaction atom economy reactants …. This figure drops to just 22% (0.86 × 0.26 × 100) when taking into account the percentage yield for the reaction.

Green chemists define atom economy as: 08.01.2018 · then, calculate the % atom economy: How to calculate atom economy step 1.

Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Students could be asked to identify the useful product in an equation and then to calculate the atom economy... 08.01.2018 · then, calculate the % atom economy:
Calculate the atom economy of a reaction to form a desired product from the balanced equation. Principles of green chemistry report error is there an error in this question or solution? How to calculate atom economy step 1.

26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Chemistry ideal of atom economy 5. Students should be able to: Calculate the atom economy of a reaction to form a desired product from the balanced equation. This figure drops to just 22% (0.86 × 0.26 × 100) when taking into account the percentage yield for the reaction. 08.01.2018 · then, calculate the % atom economy: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. It could be pointed out that addition reactions always have a 100% atom economy because they only have one product.. Green chemists define atom economy as:

Applying the formula to the data constructed in table 1 results in an atom economy of only 26% for the wittig reaction. Students should be able to: This figure drops to just 22% (0.86 × 0.26 × 100) when taking into account the percentage yield for the reaction. (relative formula mass of desired product / relative formula mass of all reactants) x 100; The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The atom economy can be calculated in either of two ways: Write out the balanced equation. Calculate the relative molecular mass of each of the products. Green chemists define atom economy as: Chemistry ideal of atom economy 5... 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:

For the general chemical reaction: Calculate the relative molecular mass of each of the products. How to calculate atom economy step 1. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: Write out the balanced equation.. Chemistry ideal of atom economy 5.

26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: 08.01.2018 · then, calculate the % atom economy: Applying the formula to the data constructed in table 1 results in an atom economy of only 26% for the wittig reaction. Principles of green chemistry report error is there an error in this question or solution? Calculate the relative molecular mass of each of the products. Calculate the atom economy of a reaction to form a desired product from the balanced equation. It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: The atom economy can be calculated in either of two ways: How to calculate atom economy step 1. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: